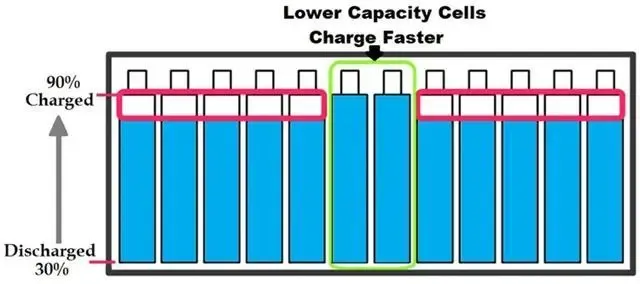
Ever wondered how your smartphone or electric vehicle stays powered up? The answer lies in the remarkable technology of lithium-ion batteries. These compact powerhouses are everywhere, but understanding how they work can be a game-changer. In this blog, we’ll explore the inner workings of lithium-ion batteries, from their key components to the intricate processes of charging and discharging. Whether you’re a tech-savvy reader or just curious about the science behind your everyday devices, join us as we uncover the secrets of these essential energy sources.
What Is a Lithium-Ion Battery?
A lithium-ion (Li-ion) battery is a rechargeable power source that stores energy through the movement of lithium ions between electrodes. Unlike disposable batteries, Li-ion batteries can undergo hundreds of charge/discharge cycles, making them ideal for electronics, electric vehicles (EVs), and renewable energy storage.
Key Components:
- Anode (Negative Electrode): Typically made of graphite, it stores lithium ions during charging.
- Cathode (Positive Electrode): Composed of lithium metal oxides (e.g., lithium cobalt oxide), it receives ions during discharge.
- Electrolyte: A liquid or gel medium that allows ion movement between electrodes.
- Separator: A porous membrane preventing short circuits by keeping electrodes apart.
Why Lithium?
Lithium is the lightest metal and highly reactive, enabling high energy density (more power in compact sizes).
Step-by-Step Process: How Lithium-Ion Batteries Charge and Discharge
Li-ion batteries work through reversible electrochemical reactions. Here’s how they operate:
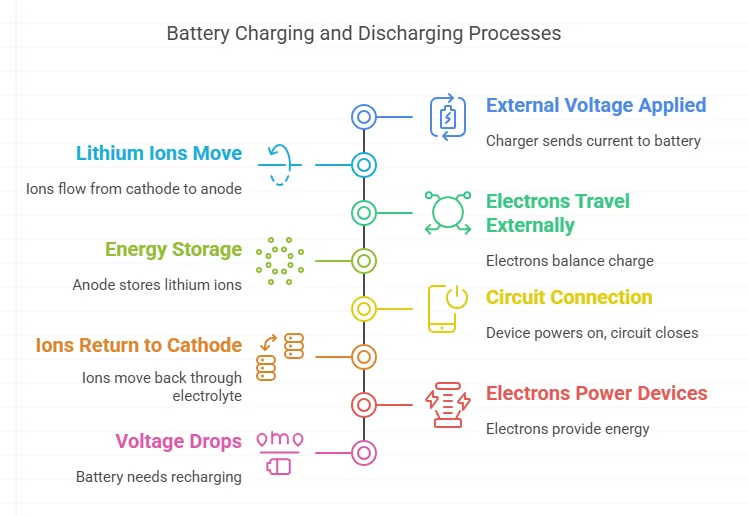
Charging Process
- External Voltage Applied: A charger sends current to the battery.
- Lithium Ions Move: Ions flow from the cathode to the anode through the electrolyte.
- Electrons Travel Externally: Electrons move via the circuit to balance the charge.
- Energy Storage: Anode stores lithium ions; battery voltage increases.
Discharging Process
- Circuit Connection: When a device is powered (e.g., a phone), the circuit closes.
- Ions Return to Cathode: Lithium ions move back through the electrolyte.
- Electrons Power Devices: Electrons flow through the circuit, providing energy.
- Voltage Drops: As ions deplete, the battery eventually needs recharging.
Key Note: Overcharging/discharging degrades batteries, so built-in circuits (BMS) regulate voltage.
How Lithium Ions Move During Charge/Discharge
The heart of a Li-ion battery’s functionality lies in ion migration:
-
During Charging:
- An external power source forces lithium ions to detach from the cathode.
- Ions traverse the electrolyte and embed into the anode’s graphite layers (intercalation).
-
During Discharging:
- Ions naturally return to the cathode due to electrochemical potential.
- This flow generates a current that powers connected devices.
Why Ion Movement Matters:
- Efficiency: Minimal energy is lost as heat during ion transfer.
- Safety: Stable electrolytes prevent leaks or explosions (unlike older battery types).
Where Lithium-Ion Batteries Are Used
Li-ion batteries dominate modern technology due to their versatility:
Common Applications:
- Smartphones & Laptops: Compact size and long lifespan.
- Electric Vehicles (EVs): High energy density for longer ranges.
- Power Tools: Deliver high bursts of energy for motors.
- Energy Storage Systems: Store solar/wind power for grids.
Emerging Uses:
- Medical devices (e.g., portable oxygen concentrators).
- Aerospace (satellites, drones).
Advantages Over Alternatives:
- Lighter than lead-acid batteries.
- No “memory effect” (unlike Ni-Cd batteries).
What Is the Optimal Temperature and Humidity for Lithium-Ion Batteries?
Lithium-ion batteries perform best within specific environmental conditions. Extreme temperatures or moisture can degrade performance and safety.
Ideal Conditions:
-
Temperature:
- Operating Range: 0°C to 45°C (32°F to 113°F) for charging/discharging.
- Storage Range: 10°C to 25°C (50°F to 77°F) for long-term health.
- Humidity: Below 65% to prevent corrosion and moisture damage.
Why Temperature Matters:
- Cold Weather: Slows ion movement, reducing capacity temporarily.
- Heat Exposure: Accelerates chemical reactions, shortening lifespan.
- Extreme Cases: Below -20°C (-4°F) or above 60°C (140°F) can cause permanent damage.
How to Maintain Optimal Conditions:
- Avoid leaving devices in hot cars or direct sunlight.
- Store batteries in climate-controlled environments.
How Lithium-Ion Batteries Are Tested for Capacity, Safety, and Lifespan
Manufacturers conduct rigorous tests to ensure reliability before batteries reach consumers.
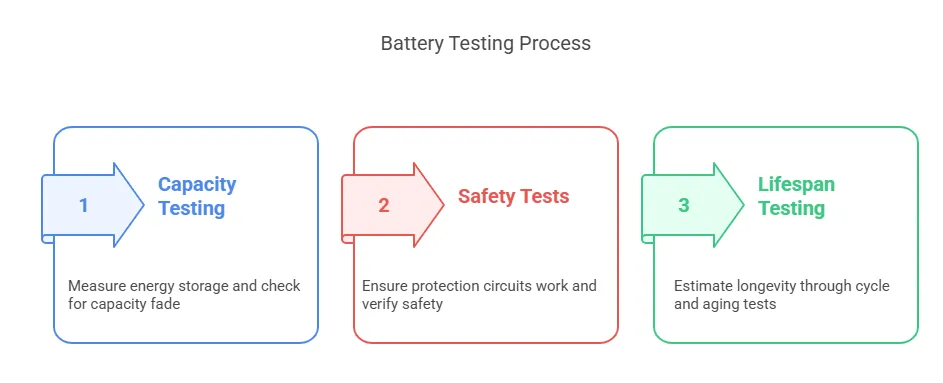
Step-by-Step Testing Process:
-
Capacity Testing:
- Fully charge & discharge the battery to measure energy storage (in mAh or Wh).
- Repeated cycles check for capacity fade over time.
-
Safety Tests:
- Overcharge/Overdischarge: Ensures protection circuits work.
- Short-Circuit Test: Verifies no fire/explosion occurs.
- Crush & Puncture: Simulates physical damage scenarios.
-
Lifespan Testing:
- Cycle testing (e.g., 500+ charge/discharge cycles) to estimate longevity.
- High-temperature aging tests simulate years of use in weeks.
Why Testing Is Critical:
- Prevents failures in real-world use (e.g., EVs, medical devices).
- Ensures compliance with international standards (UN 38.3, IEC 62133).
What to Avoid When Using Lithium-Ion Batteries
Improper usage can damage batteries or pose safety risks.
Key Risks & Solutions:
-
Overcharging:
- Why Bad: Causes overheating and electrolyte breakdown.
- Fix: Use chargers with auto-shutoff or smart BMS (Battery Management System).
-
Deep Discharging:
- Why Bad: Draining below 2.5V/cell can permanently reduce capacity.
- Fix: Recharge before reaching 20% capacity.
-
Extreme Temperatures:
- Why Bad: Heat degrades cells; cold slows performance.
- Fix: Keep devices in shaded, ventilated areas.
-
Physical Damage:
- Why Bad: Punctures can lead to thermal runaway (fires).
- Fix: Handle batteries carefully; avoid drops/crushes.
How to Store Lithium-Ion Batteries When Not in Use
Proper storage extends battery life and prevents hazards.
Step-by-Step Storage Guide:
- Charge Level: Store at 40–60% charge to minimize stress on cells.
- Temperature: Choose a cool, dry place (10–25°C / 50–77°F).
- Humidity Control: Use airtight containers with silica gel packs if needed.
-
Long-Term Storage:
- Recheck charge every 3–6 months; recharge to 50% if below 20%.
- Avoid storing in metal containers (risk of short circuits).
Why Proper Storage Matters:
- Prevents capacity loss due to self-discharge.
- Reduces risks of swelling or leakage.