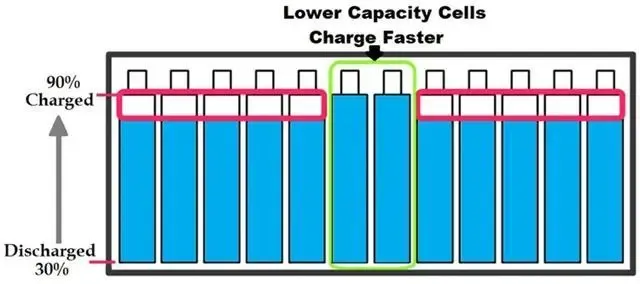
Lithium-ion batteries are the unsung heroes of our digital age, powering everything from smartphones to electric vehicles. But what exactly makes them so essential? In this article, we’ll break down the basic concepts of lithium-ion batteries, exploring their unique chemistry, high energy density, and the absence of memory effect. We’ll also take a closer look at how they are manufactured, from electrode preparation to final testing, and why maintaining a clean environment is crucial. Whether you’re curious about the science behind your device or interested in the broader applications of lithium-ion technology, this article will provide a comprehensive overview of why these batteries are so dominant in today’s world.
What Is a Lithium-Ion Battery? Basic Concepts Explained
Lithium-ion (Li-ion) batteries are rechargeable power sources that store energy through the movement of lithium ions between electrodes. Here’s what makes them unique:
- Chemistry: They use lithium compounds as the anode (typically graphite) and cathode (e.g., lithium cobalt oxide).
- Energy Density: Li-ion batteries pack more energy per unit weight than alternatives like lead-acid.
- No Memory Effect: Unlike older batteries, they don’t lose capacity if charged partially.
Why Lithium?
Lithium is the lightest metal and highly reactive, enabling efficient energy transfer. Combined with non-aqueous electrolytes, it allows for stable, high-voltage operation.
Key Components:
- Anode: Releases electrons during discharge.
- Cathode: Accepts electrons.
- Electrolyte: Facilitates ion movement.
- Separator: Prevents short circuits.
How Are Lithium-Ion Batteries Manufactured? A Step-by-Step Workflow
Step 1: Electrode Preparation
- Anode: Graphite slurry is coated onto copper foil.
- Cathode: Lithium metal oxide (e.g., NMC) is applied to aluminum foil.
Step 2: Drying and Calendering
- Electrodes are dried and compressed to ensure uniform thickness.
Step 3: Assembly
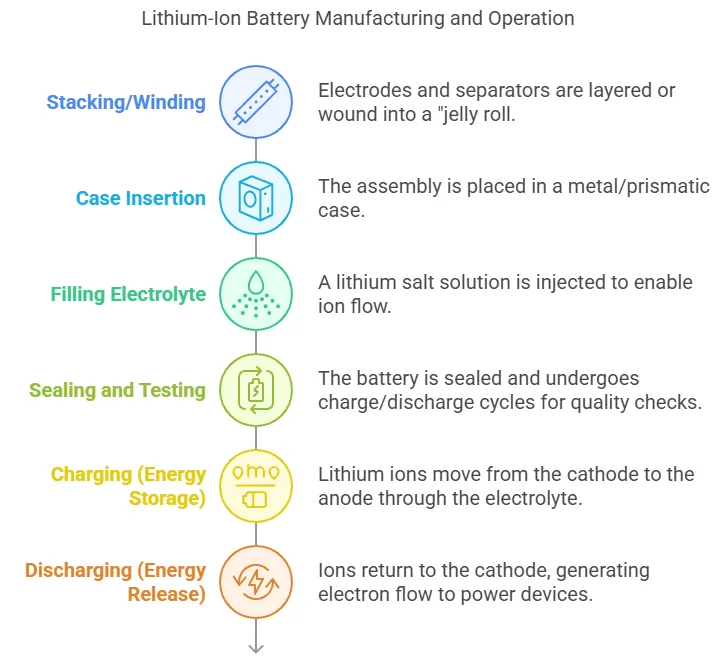
- Stacking/Winding: Electrodes and separators are layered or wound into a “jelly roll.”
- Case Insertion: The assembly is placed in a metal/prismatic case.
Step 4: Filling Electrolyte
- A lithium salt solution is injected to enable ion flow.
Step 5: Sealing and Testing
- The battery is sealed and undergoes charge/discharge cycles for quality checks.
Critical Note:
- Cleanrooms are essential to avoid contamination.
- Moisture control is vital (electrolytes react with water).
How Does a Lithium-Ion Battery Work?
Charge/Discharge Cycle Explained:
-
Charging (Energy Storage):
- Lithium ions move from the cathode to the anode through the electrolyte.
- Electrons flow via the external circuit (charging device).
-
Discharging (Energy Release):
- Ions return to the cathode, generating electron flow to power devices.
Why It’s Efficient:
- The process is reversible with minimal energy loss (~80–90% round-trip efficiency).
Safety Mechanisms:
- Built-in circuits prevent overcharging/overheating.
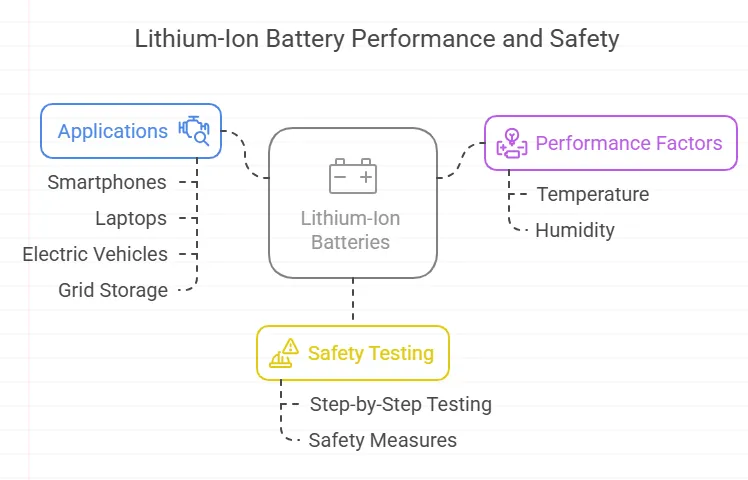
Lithium-Ion Battery Applications: From Smartphones to EVs
Where You’ll Find Li-ion Batteries:
-
Consumer Electronics:
- Smartphones, laptops (high energy density suits compact designs).
- Wireless earbuds (fast rechargeability).
-
Transportation:
- Electric vehicles (Tesla, BYD use NMC/LFP batteries).
- E-bikes (lightweight for portability).
-
Industrial/Energy Storage:
- Grid storage (stores solar/wind energy).
- Medical devices (reliable, long-life cycles).
Why Dominant?
- Longer lifespan (500–1,500 cycles) vs. NiMH batteries.
- Environmentally safer (no liquid cadmium/lead).
How Temperature and Humidity Affect Lithium-Ion Battery Performance
Why Temperature Matters
Lithium-ion batteries operate optimally within a 10°C to 35°C (50°F to 95°F) range. Extreme conditions degrade performance:
-
High Temperatures (>45°C / 113°F):
- Accelerate chemical reactions, causing faster capacity loss.
- Increase risk of thermal runaway (dangerous overheating).
-
Low Temperatures (<0°C / 32°F):
- Slow ion movement, reducing power output.
- Temporary capacity loss (up to 50% at -20°C).
Humidity’s Hidden Impact
- Moisture reacts with electrolytes, forming hydrofluoric acid (HF), which corrodes battery components.
- High humidity during manufacturing can lead to defects (e.g., internal short circuits).
How to Mitigate Risks:
✔ Store batteries at 20–25°C (68–77°F) with <60% humidity.
✔ Avoid leaving devices in hot cars or direct sunlight.
✔ Use battery management systems (BMS) to regulate temperature.
How Lithium-Ion Batteries Are Tested for Safety and Reliability
Step-by-Step Testing Process
-
Electrical Testing
- Cycle Life Test: Charge/discharge 500+ times to measure capacity fade.
- Overcharge/Discharge Test: Ensures safety circuits prevent explosions.
-
Environmental Stress Tests
- Thermal Shock: Expose to -40°C to +85°C repeatedly.
- Crush Test: Simulate physical damage (UL1642 standard).
-
Safety Abuse Tests
- Nail Penetration: Piercing the battery to trigger thermal runaway.
- Short Circuit Test: Check if protective mechanisms activate.
Why Testing Is Critical
- Consumer safety: Prevents fires (e.g., Samsung Galaxy Note 7 recall).
- Regulatory compliance: UN38.3, IEC 62133 certifications are mandatory for shipping.
How to Prevent Lithium-Ion Battery Overheating or Swelling
Common Causes of Overheating/Swelling:
- Overcharging (exceeds 4.2V per cell).
- Physical damage (punctures, drops).
- Poor ventilation (e.g., laptops on soft surfaces).
Prevention Steps:
✅ Use OEM Chargers Only
- Cheap chargers may lack voltage regulation.
✅ Avoid Extreme Temperatures
- Never charge below 0°C or above 45°C.
✅ Monitor Battery Health
- Replace if capacity drops below 80%.
✅ Store Properly
- Keep at 40–60% charge for long-term storage.
What to Do If a Battery Swells:
- Stop using it immediately.
- Place in a fireproof container.
- Recycle at a certified facility (do not puncture!).
How to Spot and Handle Damaged Lithium-Ion Batteries
Warning Signs of Damage:
⚠️ Physical Deformities: Swelling, leaks, or dents.
⚠️ Performance Issues: Rapid discharge, overheating.
⚠️ Odor/Smoke: Sweet chemical smell (electrolyte leak).
Step-by-Step Handling Guide:
-
Isolate the Battery
- Move away from flammable materials.
-
Use Protective Gear
- Wear gloves/goggles (electrolyte is toxic).
-
Dispose Safely
- Take to a hazardous waste recycling center (do not trash!).
Why Proper Handling Matters
- Damaged batteries can combust spontaneously.
- Toxic chemicals (e.g., cobalt, lithium) harm the environment if landfilled.
This article offers a detailed look at lithium-ion batteries, starting with their fundamental concepts and unique chemistry. It explains how lithium compounds are used in the anode and cathode, and how these batteries store energy through the movement of lithium ions. The manufacturing process is outlined step-by-step, from electrode preparation and drying to assembly and electrolyte filling, emphasizing the importance of cleanrooms and moisture control. The article also covers the charge/discharge cycle, applications in consumer electronics, transportation, and energy storage, and how temperature and humidity impact performance. Safety mechanisms, including built-in circuits and rigorous testing procedures, are discussed to ensure consumer protection. Finally, tips for preventing overheating, handling damaged batteries, and proper disposal are provided to highlight the importance of responsible battery management.