In the quest for sustainable and cost-effective energy storage solutions, sodium-ion batteries (SIBs) have emerged as a promising alternative to traditional lithium-ion batteries. With the increasing demand for renewable energy and electric vehicles, the need for efficient and affordable batteries has never been more critical. This article delves into the world of sodium-ion batteries, exploring their structure, applications, advantages, and challenges. From renewable energy storage to electric vehicles, SIBs are poised to revolutionize the way we power our world.
What is a Sodium-Ion Battery?
A sodium-ion battery (SIB) is a type of rechargeable battery that uses sodium ions (Na+) as charge carriers. Similar to lithium-ion batteries, sodium-ion batteries store and release energy through the movement of ions between the cathode and anode during charging and discharging cycles. However, instead of relying on lithium, sodium-ion batteries utilize sodium, which is more abundant and cost-effective. This makes SIBs a promising alternative for large-scale energy storage and applications where cost and resource availability are critical factors.
Why is the Structure and Composition of Sodium-Ion Batteries Important?
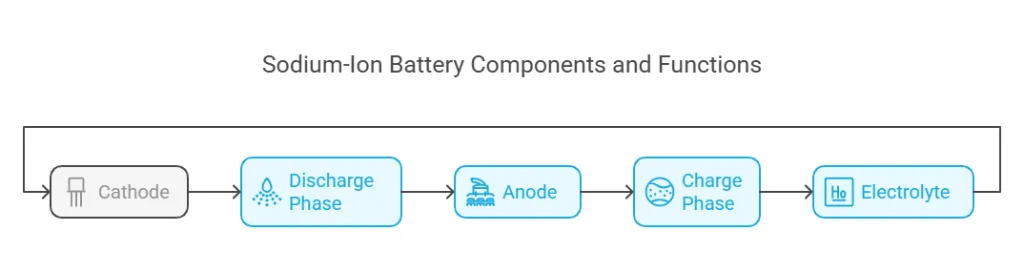
The structure and composition of sodium-ion batteries play a crucial role in their performance, efficiency, and safety. A typical sodium-ion battery consists of three main components:
- Cathode: Often made of layered transition metal oxides, polyanionic compounds, or Prussian blue analogs, the cathode is responsible for hosting sodium ions during discharge.
- Anode: Commonly composed of hard carbon, titanium-based materials, or alloy compounds, the anode stores sodium ions during charging.
- Electrolyte: A liquid or solid medium that facilitates the movement of sodium ions between the cathode and anode. It is usually a sodium salt dissolved in an organic solvent.
The choice of materials for these components directly impacts the battery’s energy density, cycle life, and thermal stability. Researchers are continuously exploring new materials and designs to optimize the structure and composition of sodium-ion batteries for better performance.
How Do Sodium-Ion Batteries Work?
The working principle of sodium-ion batteries is based on the movement of sodium ions between the cathode and anode during charging and discharging. Here’s a step-by-step breakdown of the process:
-
Discharging (Using the Battery):
- Sodium ions move from the anode to the cathode through the electrolyte.
- Electrons flow through the external circuit, providing electrical energy to the device.
- The cathode material stores the sodium ions.
-
Charging (Recharging the Battery):
- An external voltage is applied, forcing sodium ions to move back to the anode.
- Electrons flow back through the external circuit to the anode.
- The anode material stores the sodium ions for the next discharge cycle.
This reversible process allows sodium-ion batteries to be recharged and used repeatedly, making them a sustainable energy storage solution.
What Are the Applications of Sodium-Ion Batteries in Various Industries?
Sodium-ion batteries are gaining traction across multiple industries due to their cost-effectiveness and sustainability. Some key applications include:
- Renewable Energy Storage: SIBs are ideal for storing energy generated from solar panels and wind turbines, ensuring a stable power supply even when renewable sources are intermittent.
- Electric Vehicles (EVs): While still in the early stages, sodium-ion batteries are being explored as a cheaper alternative to lithium-ion batteries for EVs, especially in regions where cost is a major concern.
- Grid Storage: Sodium-ion batteries can be used for large-scale energy storage to balance supply and demand on the electrical grid.
- Consumer Electronics: With advancements in energy density, SIBs could power devices like laptops, smartphones, and power tools.
- Industrial Equipment: SIBs are suitable for powering heavy machinery and backup power systems in industries.
Sodium-Ion Batteries vs. Lithium-Ion Batteries: Key Differences
When comparing sodium-ion batteries (SIBs) and lithium-ion batteries (LIBs), several key differences stand out:
-
Raw Material Availability:
- Sodium is far more abundant and widely distributed than lithium, making SIBs more cost-effective and sustainable.
-
Energy Density:
- LIBs generally have a higher energy density, meaning they can store more energy per unit weight or volume. However, SIBs are catching up with advancements in material science.
-
Cost:
- SIBs are cheaper to produce due to the lower cost of sodium and the use of less expensive materials like aluminum for the anode current collector.
-
Safety:
- Sodium-ion batteries are considered safer than lithium-ion batteries because they are less prone to thermal runaway and overheating.
-
Environmental Impact:
- SIBs have a lower environmental footprint due to the abundance of sodium and the absence of rare or toxic materials.
-
Performance in Extreme Temperatures:
- SIBs tend to perform better at lower temperatures compared to LIBs, making them suitable for colder climates.
By understanding these differences, industries and consumers can make informed decisions about which type of battery best suits their needs.
What is the Future of Sodium-Ion Batteries in Energy Storage?
The future of sodium-ion batteries (SIBs) in energy storage looks promising, driven by their cost-effectiveness, sustainability, and scalability. As the demand for renewable energy sources like solar and wind grows, the need for efficient and affordable energy storage solutions becomes critical. Sodium-ion batteries are emerging as a strong contender due to their ability to store large amounts of energy at a lower cost compared to lithium-ion batteries. Additionally, advancements in material science and battery design are expected to improve their energy density, cycle life, and safety, making them a viable option for grid storage, residential energy systems, and industrial applications.
How Does the Charging and Discharging Process of Sodium-Ion Batteries Work?
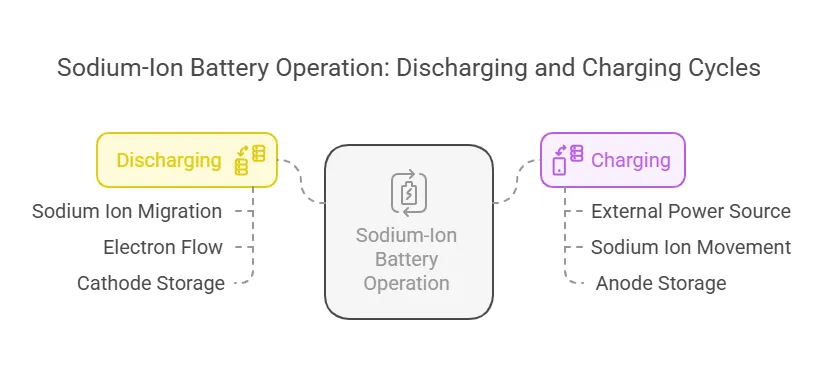
The charging and discharging process of sodium-ion batteries involves the movement of sodium ions between the cathode and anode. Here’s a step-by-step explanation:
-
Discharging (Using the Battery):
- Sodium ions migrate from the anode to the cathode through the electrolyte.
- Electrons flow through the external circuit, powering the connected device.
- The cathode material stores the sodium ions until the battery is recharged.
-
Charging (Recharging the Battery):
- An external power source applies voltage, forcing sodium ions to move back to the anode.
- Electrons return to the anode through the external circuit.
- The anode stores the sodium ions, preparing the battery for the next discharge cycle.
This reversible process ensures that sodium-ion batteries can be reused multiple times, making them a sustainable energy storage solution.
Why Are Sodium-Ion Batteries Advantageous in Renewable Energy Systems?
Sodium-ion batteries offer several advantages in renewable energy systems, making them an attractive option for storing energy generated from sources like solar and wind. Key benefits include:
- Cost-Effectiveness: Sodium is more abundant and less expensive than lithium, reducing the overall cost of battery production.
- Sustainability: The use of widely available materials minimizes environmental impact and resource depletion.
- Scalability: SIBs are well-suited for large-scale energy storage, helping to stabilize power grids and manage energy supply during peak demand.
- Safety: Sodium-ion batteries are less prone to overheating and thermal runaway, enhancing their safety in renewable energy applications.
- Performance in Varied Conditions: SIBs perform reliably across a wide range of temperatures, making them suitable for diverse climates.
These advantages position sodium-ion batteries as a key player in the transition to cleaner and more sustainable energy systems.
How Are Sodium-Ion Batteries Revolutionizing Electric Vehicles?
Sodium-ion batteries are poised to revolutionize the electric vehicle (EV) industry by offering a more affordable and sustainable alternative to lithium-ion batteries. Here’s how:
- Lower Costs: The use of sodium, which is more abundant and cheaper than lithium, significantly reduces battery production costs, making EVs more accessible to consumers.
- Resource Availability: Sodium is widely available, reducing dependency on geographically concentrated lithium reserves.
- Environmental Benefits: SIBs have a smaller environmental footprint, aligning with the global push for greener technologies.
- Improved Safety: Sodium-ion batteries are less likely to overheat or catch fire, enhancing the safety of EVs.
- Scalability for Mass Production: The lower cost and abundant materials make it easier to scale up production to meet the growing demand for EVs.
While sodium-ion batteries currently have lower energy density compared to lithium-ion batteries, ongoing research is expected to bridge this gap, making SIBs a game-changer in the EV market.
What Are the Key Factors Impacting the Performance of Sodium-Ion Batteries?
The performance of sodium-ion batteries is influenced by several key factors, including:
-
Electrode Materials:
- The choice of cathode and anode materials directly affects energy density, cycle life, and charging speed. Common materials include layered oxides for cathodes and hard carbon for anodes.
-
Electrolyte Composition:
- The electrolyte must facilitate efficient ion transport while maintaining stability. Researchers are exploring liquid, solid, and hybrid electrolytes to optimize performance.
-
Temperature Sensitivity:
- Sodium-ion batteries perform differently at various temperatures. Advances in thermal management systems are crucial to ensure consistent performance.
-
Cycle Life:
- The number of charge-discharge cycles a battery can endure before its capacity degrades is a critical factor. Improvements in electrode design and electrolyte stability can enhance cycle life.
-
Manufacturing Processes:
- Efficient and scalable manufacturing techniques are essential to reduce costs and improve the quality of sodium-ion batteries.
By addressing these factors, researchers and manufacturers can unlock the full potential of sodium-ion batteries, making them a competitive option for various applications.
What Are the Testing Methods for Sodium-Ion Battery Efficiency and Safety?
Testing the efficiency and safety of sodium-ion batteries (SIBs) is crucial to ensure their reliability and performance. Here are some common testing methods:
-
Cycle Life Testing:
- Repeatedly charging and discharging the battery to determine how many cycles it can endure before its capacity significantly degrades.
-
Energy Density Measurement:
- Evaluating the amount of energy the battery can store per unit weight or volume to assess its efficiency.
-
Thermal Stability Testing:
- Exposing the battery to high temperatures to check for risks of overheating, thermal runaway, or failure.
-
Rate Capability Testing:
- Measuring how well the battery performs under different charging and discharging rates to understand its versatility.
-
Safety Tests:
- Conducting tests like nail penetration, overcharging, and short-circuiting to evaluate the battery’s safety under extreme conditions.
-
Impedance Spectroscopy:
- Analyzing the internal resistance of the battery to identify potential issues with ion transport and electrode materials.
These tests help manufacturers and researchers identify weaknesses and improve the design and materials of sodium-ion batteries.
Why is the Environmental Impact and Sustainability of Sodium-Ion Batteries Important?
Sodium-ion batteries (SIBs) are gaining attention not only for their performance but also for their environmental benefits. Here’s why their sustainability matters:
-
Abundance of Sodium:
- Sodium is one of the most abundant elements on Earth, reducing the need for mining rare or geographically concentrated resources like lithium.
-
Lower Carbon Footprint:
- The production of sodium-ion batteries generates fewer greenhouse gas emissions compared to lithium-ion batteries, contributing to climate change mitigation.
-
Recyclability:
- Many components of SIBs, such as aluminum current collectors, are easier to recycle than those in lithium-ion batteries, promoting a circular economy.
-
Reduced Toxicity:
- Sodium-ion batteries often use less toxic materials, minimizing environmental contamination during production and disposal.
-
Support for Renewable Energy:
- By enabling efficient energy storage for solar and wind power, SIBs help reduce reliance on fossil fuels.
These factors make sodium-ion batteries a more sustainable choice for the future of energy storage.
How to Take Precautions When Using Sodium-Ion Batteries?
While sodium-ion batteries are generally safer than lithium-ion batteries, certain precautions are necessary to ensure safe usage:
-
Avoid Overcharging:
- Use chargers specifically designed for sodium-ion batteries to prevent overcharging, which can lead to overheating.
-
Monitor Temperature:
- Keep the battery within its recommended temperature range to avoid thermal stress or failure.
-
Prevent Physical Damage:
- Avoid dropping, puncturing, or crushing the battery, as physical damage can compromise its safety.
-
Store Properly:
- Store batteries in a cool, dry place away from direct sunlight or flammable materials.
-
Follow Manufacturer Guidelines:
- Always adhere to the manufacturer’s instructions for charging, discharging, and handling the battery.
By following these precautions, users can maximize the safety and longevity of sodium-ion batteries.
How to Maintain and Care for Sodium-Ion Batteries to Maximize Lifespan?
Proper maintenance and care can significantly extend the lifespan of sodium-ion batteries. Here’s a step-by-step guide:
-
Regular Charging:
- Avoid letting the battery fully discharge. Keep it between 20% and 80% charge for optimal performance.
-
Temperature Control:
- Store and use the battery in environments with moderate temperatures (typically between 15°C and 25°C).
-
Clean Contacts:
- Periodically clean the battery terminals to ensure good electrical connections and prevent corrosion.
-
Avoid Overloading:
- Do not connect devices that draw more power than the battery is designed to handle.
-
Periodic Calibration:
- Fully charge and discharge the battery every few months to recalibrate its capacity readings.
-
Inspect for Damage:
- Regularly check for signs of swelling, leakage, or other physical damage and replace the battery if necessary.
By following these maintenance tips, users can ensure their sodium-ion batteries remain efficient and durable over time.
What Are the Challenges in Scaling Sodium-Ion Battery Production?
Scaling up the production of sodium-ion batteries (SIBs) presents several challenges that need to be addressed:
-
Material Optimization:
- Identifying and sourcing cost-effective, high-performance materials for cathodes, anodes, and electrolytes remains a hurdle.
-
Manufacturing Infrastructure:
- Existing manufacturing facilities are primarily designed for lithium-ion batteries, requiring significant investment to adapt for SIB production.
-
Energy Density Limitations:
- Sodium-ion batteries currently have lower energy density compared to lithium-ion batteries, which limits their use in high-demand applications like electric vehicles.
-
Standardization:
- The lack of standardized production processes and quality control measures can lead to inconsistencies in battery performance.
-
Supply Chain Development:
- Building a robust supply chain for sodium-based materials is essential to meet the growing demand for SIBs.
-
Research and Development Costs:
- Ongoing R&D is required to improve the performance and reduce the costs of sodium-ion batteries, which can be resource-intensive.
Addressing these challenges will be critical to making sodium-ion batteries a mainstream energy storage solution.
Sodium-ion batteries (SIBs) are gaining traction as a sustainable and cost-effective energy storage solution. Unlike lithium-ion batteries, SIBs utilize abundant and inexpensive sodium, making them ideal for large-scale applications. The structure of SIBs includes a cathode, anode, and electrolyte, with materials like layered transition metal oxides and hard carbon playing crucial roles. SIBs offer several advantages, including lower costs, enhanced safety, and better performance in extreme temperatures. They are being explored for renewable energy storage, electric vehicles, grid storage, and consumer electronics. However, challenges such as lower energy density and the need for material optimization remain. As research progresses, SIBs are expected to become a game-changer in the energy storage landscape, driving the transition to a greener and more sustainable future.