What limits the charging speed of lithium ion batteries?

Follow me on:

What limits the charging speed of lithium ion batteries?
In the final analysis, it is still materials and technology. The process of fully charging a lithium ion battery is that lithium ions travel between the positive and negative electrodes, and realize the charging and discharging function of the lithium battery by carrying and releasing electrons.This requires a certain reaction time. Too fast charging will cause abnormal reaction of the lithium battery that produce crystallization, and if the charging speed exceeds the battery’s tolerance during charging, it will increase the internal resistance of the lithium battery, so that the battery will be dangerously overheated.
In the battery industry, the charge-discharge rate is usually used to describe the relationship between the charging speed and the current. The rate when the battery is fully charged in 1 hour is called 1C, and the rate when it only takes 30 minutes is called 2C, and so on, more than 1C can be called fast charging. Nowadays, the charging rate of lithium-ion batteries can generally reach 1C-3C, and some can even reach 5C. However, compared to the discharge rate of 10C, it is still far worse.
In addition to the bottleneck of the maximum charging rate, the charging rate that the battery can withstand under different SOC (State of Charge) is also different. Generally the charging rate will follow the law of slow-fast-slow, when the SOC reaches more than 90%, the internal resistance of the battery will increase significantly, which will slow down the charging rate.
So if you are an electric vehicle user and want to save as much time as possible on charging, try not to use the power below 10%, and it doesn’t have to be fully charged when charging, 90% or more or enough power that can cover miles of your next trip.
In addition to the bottleneck of the battery itself, peripheral charging devices also have their own limitations.
In theory, the charging speed can be increased by increasing the current. However, if the current is too large, the diffusion speed of lithium ions inside the battery cannot keep up with the diffusion speed of electrons, which will cause the electron-ion operation to be out of sync, that will affect the performance of the battery, and the achievable charging capacity will be correspondingly reduced, there is even a risk of fire and explosion. Therefore, in the case of not in a hurry, we recommend using slow charging, which is beneficial to prolong the battery life, and the lithium batteries is also safer. During charging, the diffusion rate of lithium ions inside a lithium battery is closely related to temperature, cathode material and structure.
Firstly, the higher the temperature, the faster the diffusion speed. However, if the temperature is too high, it will also lead to problems such as reduced battery life and reduced charging safety.If the temperature is too low, the metal lithium in the battery will be deposited, which will cause the internal short circuit of the battery, especially the LiFePO4 battery. Generally, the capacity of the comon LiFePO4 battery is only about 60-70% at 0 °C, and only 20-40% at -20 °C. Therefore, in the cold northern winter, electric vehicles must have the function of heating the battery module, and the power consumption is obviously faster.
The second is the material. The diffusivity of different materials is very different. LiCoO2, LiMn2O4, LiFePO4, NCM, NCA, etc. are all cathode materials with good performance.
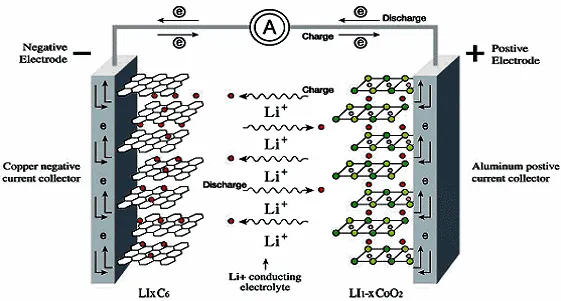
Basic working principle and structure of lithium-ion battery
Basic principle: The positive electrode undergoes a reduction reaction to gain electrons; the negative electrode undergoes an oxidation reaction and loses electrons. The electrons pass through the load and flow from the negative electrode to the positive electrode, forming a current in the direction from the positive electrode to the negative electrode.
1 (+1) valent lithium ion <—— (1-x) (+1/(1-x)) valent lithium ion + x (+1) valent lithium ion + x electrons.
Assuming x=0.5, we get:
1 (+1) valence lithium ion <——0.5 (+2) valence lithium ion + 0.5 (+1) valence lithium ion + 0.5 electrons.
Multiply both sides by 2 to get:
2 (+1) valent lithium ions <——1 (+2) valent lithium ion + 1 (+1) valent lithium ion + 1 electron.
Simplified to get:
1 (+1) valence lithium ion <——1 (+2) valence lithium ion + 1 electron.
This formula actually describes the overall reaction, not the individual reactions.
The lithium atoms of the negative electrode lose electrons and are oxidized to (+1) valence lithium ions. The electrons flow from the negative electrode into the load circuit, and the lithium ions flow to the positive electrode through the electrolyte;
The core of the positive electrode is a (+1/(1-x)) valence lithium ion, and the core of the negative electrode is a lithium atom, the two react to generate a (+1) valence lithium atom, and the flow of electrons in the redox reaction forms an electric current.
When process in lithium battery manufacturing, substances are always needed to carry the lithium ions of the positive electrode and the lithium atoms of the negative electrode, just as the goods always need shelves, then the shelf of lithium ions is phosphate ions, which together with lithium ions form the positive electrode. The lithium atoms of the negative electrode are composed of materials such as porous graphite, which will not cause the negative electrode to decrease or even disappear after the reaction. Between the positive electrode and the negative electrode is an electrolyte and separator, which is used not only for the flow of lithium ions, but also for isolating the positive and negative electrodes to prevent internal short circuits.

Lithium-ion battery characteristics
The characteristic of lithium-ion batteries that users are most concerned about is the capacity, such as 2000mAh, which refers to the number of charges that the lithium-ion battery can release under normal working conditions.
Let’s look at a specification sheet of a lithium-ion battery. The more important parameters of this battery are:
Capacity: 2000 mAh
Charge cut-off voltage: 4.2 V
Discharge cut-off voltage: 2.5 V
Maximum charging current: 4000 mA
Maximum discharge current: 20000 mA
In short, it is all about battery capacity and charging and discharging. Battery capacity depends on how many electrons the negative electrode can release and how many electrons the positive electrode can receive.
Why is there a charge cut-off voltage?
In other words, what are the consequences of overvoltage charging? The negative electrode is composed of graphite and lithium atoms. In fact, lithium does not exist in the form of atoms, but coexists with graphite in the form of lithium ions. After overvoltage charging, lithium ions will be precipitated into crystalline lithium, and crystalline lithium cannot participate in charging and discharging, which will reduce the battery capacity.
Why is there a discharge cut-off voltage?
In other words, what are the consequences of overvoltage discharging? After over-discharge, a large amount of lithium ions in the negative electrode flows to the positive electrode, resulting in emptiness of the graphite and collapse of some areas. The collapsed area can no longer store lithium ions, which will also reduce the battery capacity.
Welcome to discuss lithium battery making with us, our email is sales08@sztaipu.com.